祝贺2020级直博生岳斌关于分子筛捕获N2O的论文,“Efficient nitrous oxide capture by cationic forms of FAU and CHA zeolites”,在Chemical Engineering Journal发表。
Abstract
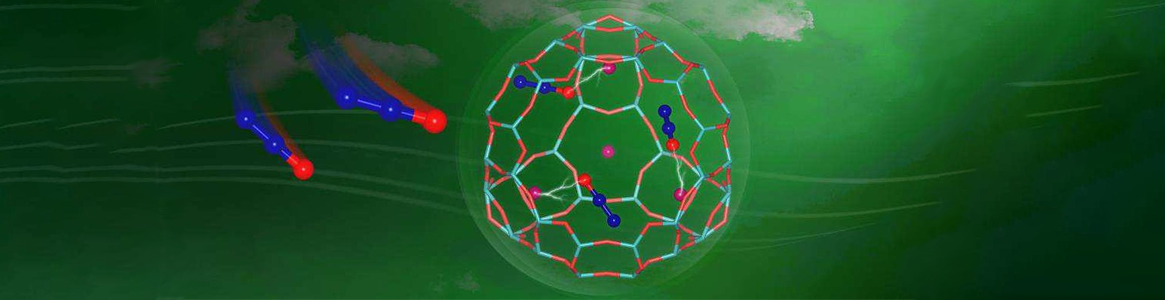
Nitrous oxide is a strong greenhouse gas contributing over 300 times global warming potential higher than carbon dioxide. The capture of nitrous oxide from industrial emission source represents an efficient and promising strategy for its further treatment or recycling, which receives less attention in the past decades. Herein, a comprehensive study on nitrous oxide capture by alkali and alkaline-earth metal ions (Li, Na, K, Mg, Ca and Ba) exchanged FAU and CHA zeolites was conducted, with focuses on the underlying adsorption mechanism and the structure-performance relationship. Under ambient conditions, Ba-FAU and Na-CHA show remarkable nitrous oxide dynamic uptakes of 2.25 and 2.41 mmol/g, respectively, at high concentration of 50000 ppm, and Ba-FAU shows good dynamic uptake of 0.35 mmol/g at extremely low concentration of 1000 ppm. The dynamic nitrous oxide uptakes by zeolites at high concentrations are related to the surface area and the adsorption strength controlled by local electrostatic field, while the diffusion resistance shows serious negative impacts at low concentrations. The intrinsic stability of zeolite and the perfect recyclability make Ba-FAU an eligible industrial adsorbent for nitrous oxide capture. In situ Fourier transform infrared spectroscopy and Grand Canonical Monte Carlo simulations reveal that nitrous oxide molecules prefer to anchor to extraframework cations via terminal-oxygen atoms and meanwhile stabilized by framework oxygen through electrostatic interaction via central nitrogen atoms. The explicit structure-performance relationship and the underlying mechanism are very important for the understanding of nitrous oxide capture by zeolites and most helpful for the rational design of robust adsorbents.
